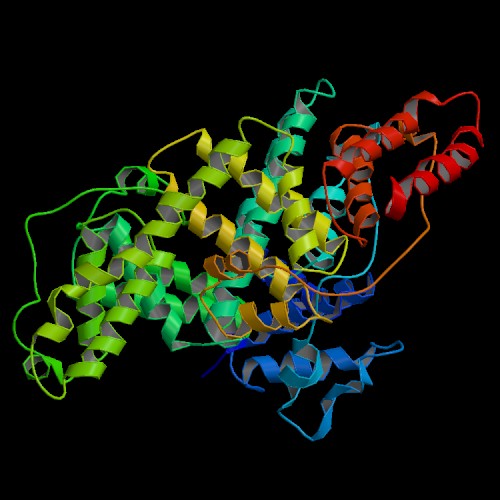

Structure determined by He and Carter (1), Protein Data Bank entry 1UOR.
To view the structure of the
asymmetric unit using interactive 3D software,
click on this link to the Protein Data Bank:
1UOR
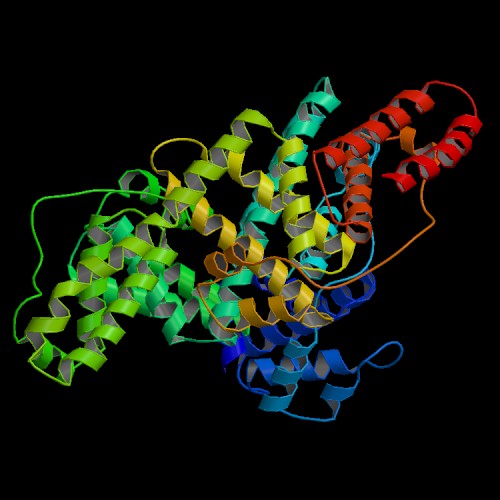
Structure determined by Sugio et al. (2), Protein Data Bank entry 1AO6.
To view the structure of the asymmetric unit using interactive 3D software,
click on this link to the Protein Data Bank:
1AO6
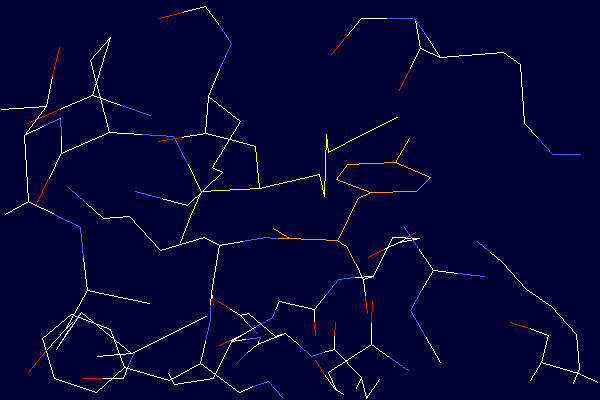
Detailed view demonstrating the close proximity of the Lysine - 525 side chain
(shown in yellow) to the aromatic ring of Tyrosine - 401 (shown in orange). From the
structure determined by He and Carter (1), Protein Data Bank entry 1UOR. Deprontonated
epsilon-amino groups of lysine side chains are known to quench the fluorescence signal
originating from nearby tyrosine residues. Therefore, deprotonated lysine residues in
close proximity to tyrosines are candidate quenching groups. Data from tyrosine
fluorescence emission studies (3) in combination with the crystal structure provide
experimental evidence suggesting the existence of lysine residues in albumin with unusually low
pKa values.
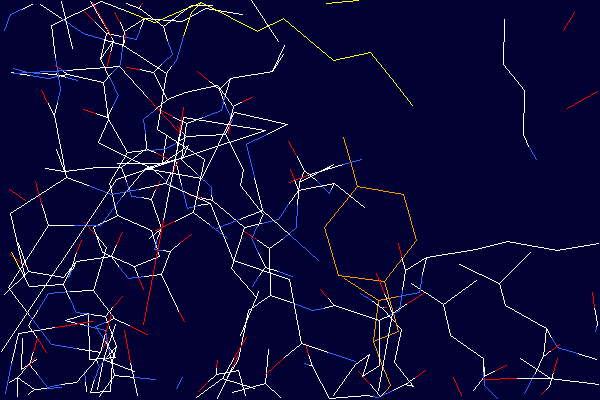
Detailed view demonstrating the close proximity of the Lysine - 525 side chain
(shown in yellow) to the aromatic ring of Tyrosine - 401 (shown in orange). From the
structure determined by Sugio et al. (2), Protein Data Bank entry 1A06. The distance
between the nitrogen atom of the Lysine - 525 side chain and the oxygen atom of the
Tyrosine - 401 side chain is 4.2 Ångstroms. Deprontonated epsilon-amino groups of lysine
side chains are known to quench the fluorescence signal originating from nearby tyrosine
residues. Therefore, deprotonated lysine residues in close proximity to tyrosines are
candidate quenching groups. Data from tyrosine fluorescence emission studies (3) in
combination with the crystal structure provide experimental evidence suggesting the existence of
lysine residues in albumin with unusually low pKa values.